First statistics for the Brazilian Fast Tack examination of COVID-19 inventions
- 17 June 2020
- Articles
Considering the pandemic declared by the World Health Organization, its impacts on public health, and the need for short, medium and long term measures to protect the population from the COVID-19 virus, the BRPTO launched on April 07, 2020 a fast-track examination procedure for patent applications covering inventions related to pharmaceutical products and processes even or materials that can be used on the diagnosis, prophylaxis and treatment of the symptoms of the COVID-19 ”.
It is important to notice that to take part of this fast-track examination program, the patent application does not need to explicitly make reference to the new virus, provided that the applicant presents a clarification that duly explain the relationship of the claimed matter with the treatment of COVID-19 symptoms. Such clarification will be duly evaluated by the patent board of examiners and then a decision of allowing or not the fast-track procedure will be issued.
This modality of fast-track procedure comprises two routes of requesting the prioritization for COVID-19 related inventions:
(i) the request may be filed by the Applicant or by any third-party who has a legitimate interest on the fast examination of a certain application; and
(ii) the request may be filed by the Ministry of Health considering that certain invention is qualified as extremely relevant for the public interest.
The first results of such initiative were recently published and the results can be observed as exposed below:
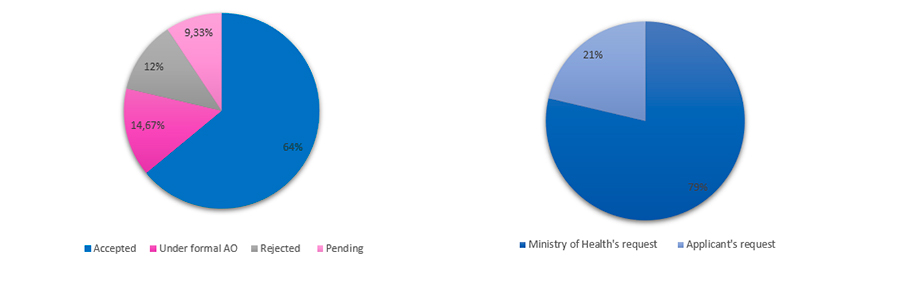
- Type of request: the majority of the fast-track procedures were requested by the Ministry of Health (59 requests representing 79% of the total number of requests). Su
- ch requests were mainly for inventions related to the drugs Favipiravir, Tocilizumab, Sarilumab and Remdesivir for being considered strategic for the Brazilian Unified System of Health (SUS) during the pandemic.
- From such 59 requests, 46 requests were already accepted by the BRPTO for the fast-track procedure until now, being composed by patent applications originated from the United States, Japan, Switzerland, France and Germany. Some of the prioritized drugs are from Gilead, Roche, Genentech, Novartis, Toyama Chemical CO., Chugai Seiyaku Kabushiki Kaisha, Regeneron Pharmaceuticals, Selecta Biosciences among others.
- The requests made by the Applicants totalize 16 cases (21% of total number of requests) and such applicants are mainly represented by Brazilian compani
- es, Universities and sole inventors.
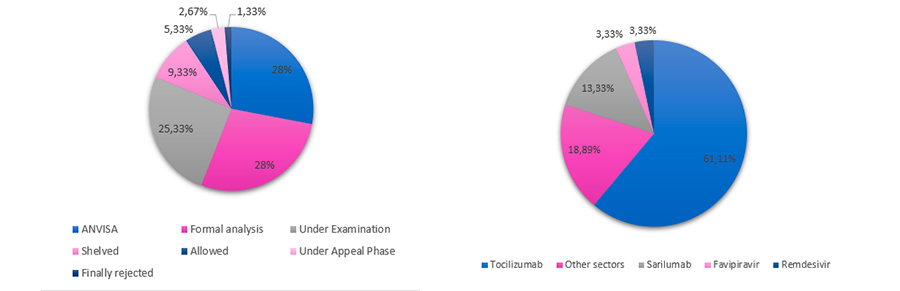
- General status of the total fast-track requests: from the 75 total requests for the fast-track procedure, until now, 48 requests (64%) were accepted (46 being from foreign companies), 11 requests (15%) are under a formal office action, 9 requests (12%) were rejected and 7 requests (9%) are still pending of a decision.
While analyzing the 46 accepted requests from foreign companies, it was possible to note that they mainly represent inventions related to Tocilizumab (37 requests – 61%). As expected, the technological areas most affected with such fast-track procedure were Chemicals (74%) and Medical devices (12%). Regarding the examination stage of the 75 requests, 21 applications (28%) are at ANVISA awaiting the prior approval and consequent return to the BRPTO, 21 applications (28%) are under formal examination, 19 applications (25%) are under technical examination and 7 applications (9%) are shelved.
Finally, it is possible to note that both BRPTO and the Ministry of Health are very active during this period of pandemic. Even being a new disease, there are many products and processes already in use that may the useful to combat the Covid-19. Therefore, it is of interest to the BRPTO to allow the fast-track, both to encourage the development of new related technologies, and to reduce the time required to issue a decision for respective patent applications.